The Ministry of Health and Population (MoHP) published a notice on 2078/01/03 regarding purchasing and use of medical devices and reagents used in the lab. The Ministry circulated a notice to use the products which are certified by regulatory bodies such as EUCE and US-FDA to ensure proper device quality. While this circulation of the Ministry is commendable, it is important to know the factors that contribute to proper patient care.
What is Medical Device?
According to WHO ‘Medical device’ means any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination, for human beings, for one or more of the specific medical purpose(s).
What is ISO?
“International Organization for Standardization,” (ISO) is a non-governmental organization based in Geneva, Switzerland.
ISO 13485 deals with standardization of medical Equipment .ISO 13485 is a stand-alone standard published by the ISO that provides requirements for quality management systems (QMS) of companies involved in the medical device industry. It is meant to ensure that medical device manufacturers set up a QMS that demonstrates consistent design, development, production, storage, distribution, installation, servicing, final decommissioning, and/or disposal of medical devices.
What is CE?

Medical devices need to adhere to the regulations of the European Union before being marketed in the EU; one of these regulations is the affixation of the Conformite ”Europe” enne (CE) marking.
CE marking ensures that devices are safe and fit for their intended use. It is seen as a proclamation by the manufacturer that the device fulfills all the designated provisions of the applicable regulation. This includes those corresponding to safety and it proves that, where needed, the device has been evaluated as per the relevant procedures. The CE mark also guarantees that the product can be freely marketed all over the EU without additional control.
What is US-FDA?

In the United States, the Federal Food Drug and Cosmetic Act regulate medical devices. Approval from Food and Drug Administration (FDA) needs to be received before marketing the medical device in the United States. The United States follows a risk-based classification for medical devices; the devices are classified according to the risk associated with the use of the device and the regulatory directives are emphasized based on classification.
FDA also emphasizes on the regulation of devices after approval. Medical device manufacturers and importers need to have processes in place to respond and report to the FDA any device-related deaths, serious injuries, or certain malfunctions.
Does CE and US-FDA ensure patient safety?
Most medical device regulations in countries manufacturing medical devices converge toward the guidance documents developed by the Global Harmonization Task Force (GHTF) and the International Medical Device Regulators Forum (IMDRF). The sole purpose of medical device regulations is to ensure the safety and performance of medical devices. However, many healthcare professionals and users of medical devices may not know the narrow scope and limitations of medical device regulations. Often, they have unrealistic expectations about the regulators ability to protect public health during the entire lifecycle of the device.
One critical factor in the regulation of medical devices in low resource countries like ours is the failure to differentiate between product safety and patient safety. At the initial stages of a medical device’s marketing and utilization lifespan, the focus should be on regulatory criteria applicable to medical devices (product safety). However, towards the middle and end stages, the proper focus of attention must be on the user responsibility and proper utilization of devices.
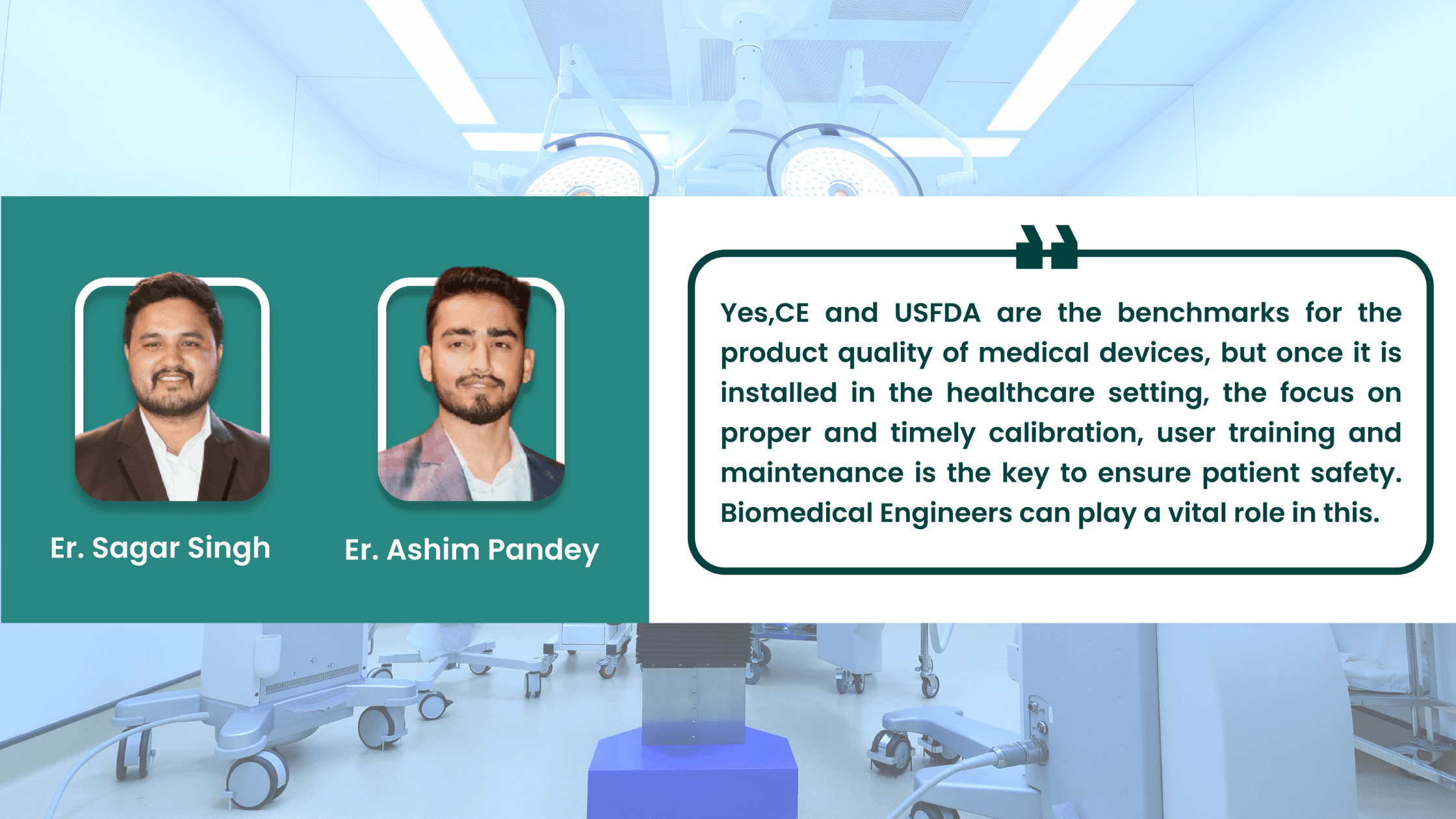
Yes, CE and USFDA are the benchmark for the product quality of medical devices but once it is installed in the healthcare setting, the focus on proper and timely calibration, user training and maintenance is the key to patient safety. Biomedical Engineers can play a vital role in this.

How should Nepal regulate medical devices?
Nepal depends entirely on imported medical devices. However, it is impractical for a medical device manufacturer to have a physical or legal presence in every country. A regulatory authority should be established that ensures basic-level controls as followed:
1. Define/Classify Medical Devices:
The regulatory authority should define the medical devices properly and classify the devices following the risk associated and make the regulatory requirements and impose international quality standards in accordance with the classification.
2. Registration of establishments
The law should require local manufacturers, authorized representatives, importers, and distributors who place medical devices on the market or make medical devices available for use in the authority, to register with the regulatory authority.
3. Listing of medical devices
The regulatory authority should establish a requirement for authorized representatives to submit a listing of medical devices they place on the national market. Representatives should be made to submit the international quality standard certification for each of the listed equipment.
4. Import controls
These may include approval of importation documents and verification of imported products. There should be mechanisms for cooperation between the regulatory authority and customs service so that medical devices will not be released from the port of entry unless there is proof that the regulatory authority has authorized them to be placed on the market.
5. Establish a system for vigilance Reporting
The regulatory authority should establish a system where users, patients, and the manufacturer of medical devices, can report complaints involving medical devices, including a malfunction at the device level and adverse events at the patient level, in particular those adverse events resulting in death or severe injury.
6. Undertake market surveillance
The regulatory authority should undertake targeted activities to ensure the proper and timely calibration, maintenance, and result validation of sensitive equipment. Authorities can ask the users/distributors to provide the detailed report of status of the devices installed within the country during the lifespan of equipment.
References:
1. Medical device regulations and patient safety, Michael Cheng.
2. WHO Global Model Regulatory Framework for Medical Devices including in vitro diagnostic medical devices.
3. Medical Device Regulations: A Current Perspective, Sandeep K Gupta.
http://4.188.83.218/static/3592.jpgarticles/relation-heart-kidney/